The tiny amplifier designed for high quality patch clamp recordings in both voltage clamp and current clamp modes.
The low-noise amplifier, the pulse generator and the digitizer are integrated within the small instrument.
ePatch
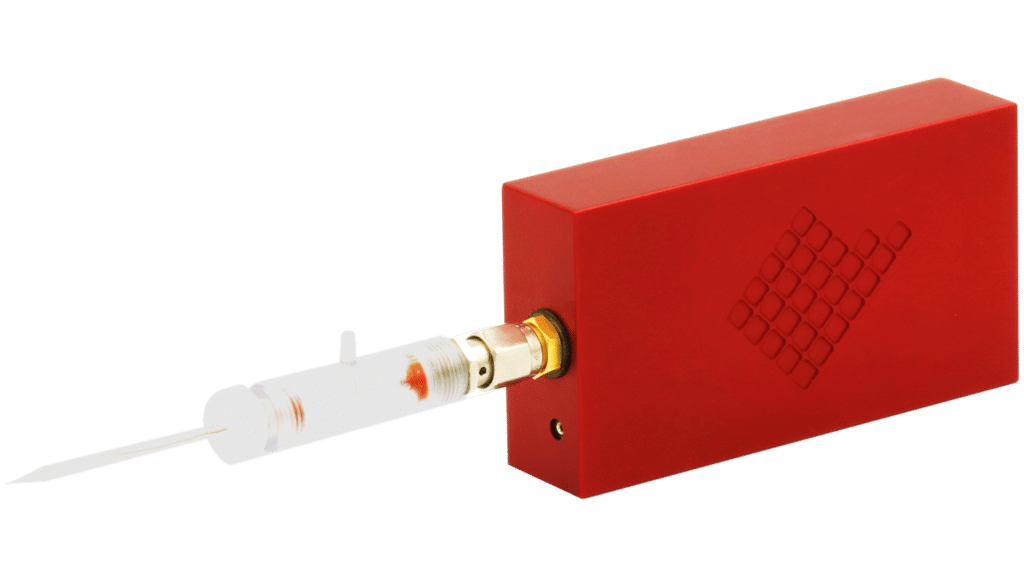
Why choose ePatch?
Miniaturized – Handheld instruments vs. bulky setup (4x2x8 cm)

Easy to use – can be directly installed to the micromanipulator mounting plate and powered by the USB port of a laptop

Affordable – we enable technologies for everyone!
Use cases
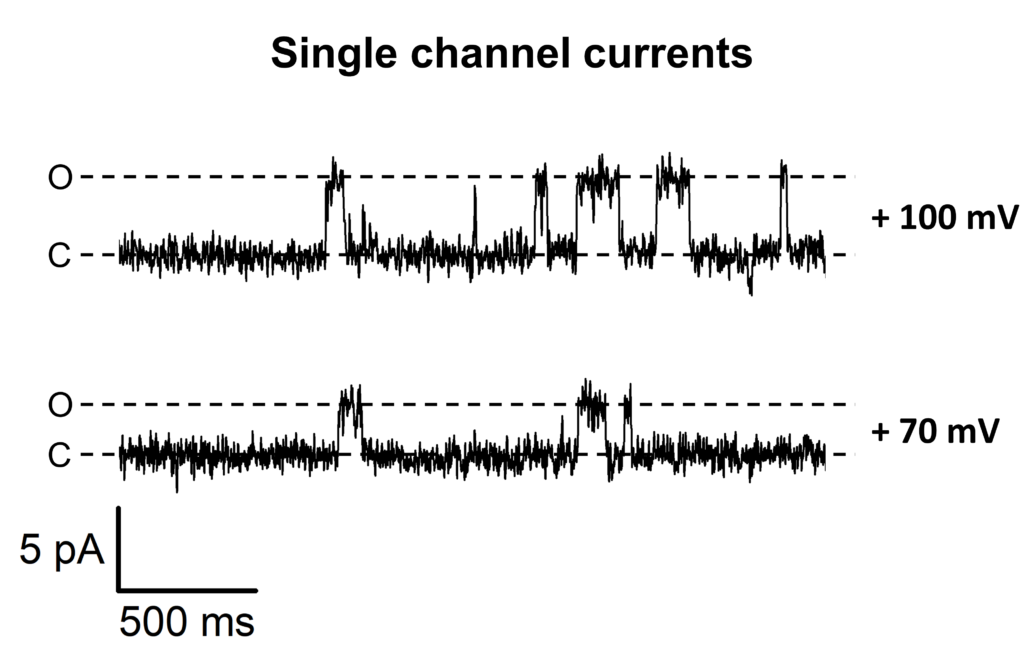
A single channel recording showing the Kcv potassium channel activity in a cell-attached patch of HEK cell membrane transiently expressing the channel. The closed and opened levels are indicated by a dotted line preceded by C or O, respectively. The pipette holding potential is indicated at the right end of the trace. K+ concentration inside the pipette was 130 mM; K+ in bath solution was 100 mM.
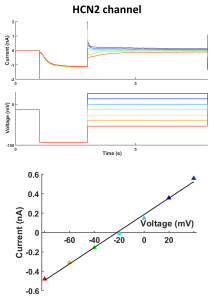
Measuring the reverse potential of HCN2 channel. HcN2 channel current activated by hyperpolarizing prepulse to -120mV, followed by a test pulse to potentials between -110 and -60 mV (top panel). Data were saved in .dat format and analyzed off-line using Mathlab software (The MathWorks, USA). Plotting the current at the beginning of the test pulse against the test pulse potential provides the instantaneous current–voltage relation. Fitting data with a linear regression yields the reversal potential of -21,88 mV (bottom panel), in agreement with the the experimental conditions (25 C°; [K+]in 130mM; [K+]out 30mM; [Na+]in 10mM; [Na+]out 110mM) and the published PNa/PK
– Biel, Martin, Christian Wahl-Schott, Stylianos Michalakis, and Xiangang Zong. 2009. “Hyperpolarization-Activated Cation Channels: From Genes to Function.” Physiological Reviews 89(3): 847–85.
.
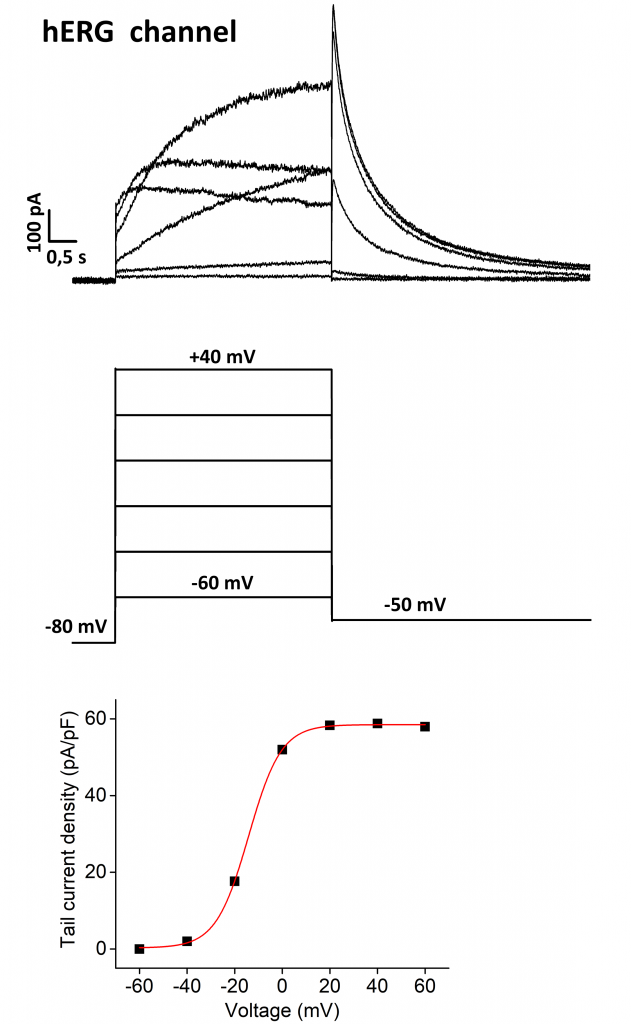
Voltage dependence of activation of hERG channel. Outward K+ current elicited by hERG channel transiently expressed in mammalian HEK293T cells (top panel). Cells were held at -80 mV and depolarized to voltages between -60 and +40 mV for 4 s, and then clamped to –50 mV for 6 s (centre panel). Data were saved in .abf format (Axon binary format v2.0) and analyzed off-line. Voltage-dependence of activation was obtained from plots of tail currents against voltage; fitting to the data with Boltzmann equation provides the half activation potential of -16,2 mV (bottom panel).
– Butler, Andrew et al. 2018. “Action Potential Clamp Characterization of the S631A HERG Mutation Associated with Short QT Syndrome.” Physiological reports 6(17)
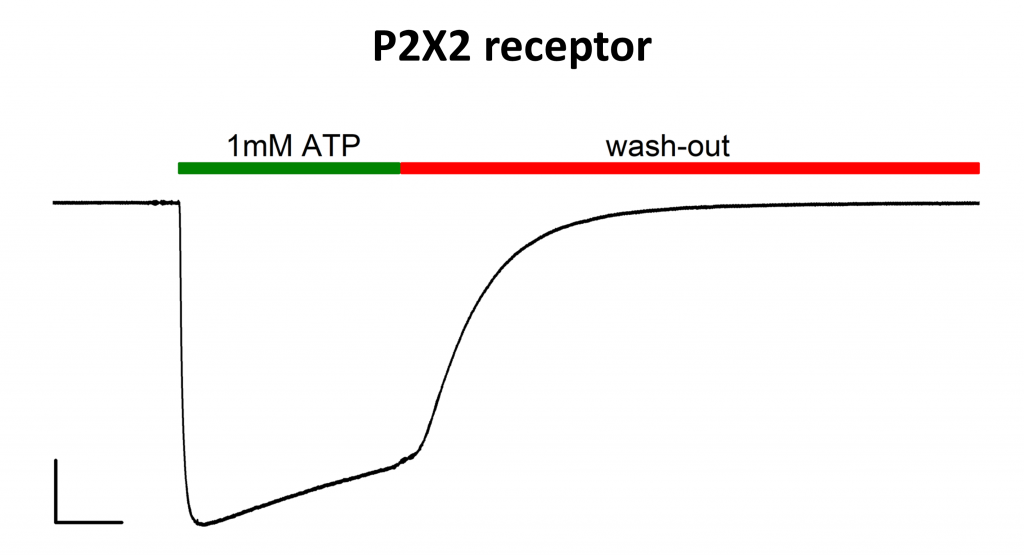
ATP-evoked inward current of P2X2 receptor transiently expressed in mammalian HEK293T cells. The cell was held at -40mV under the whole-cell configuration. Scale bar 500 ms/1nA. ATP was delivered to the cell for ~ 1,5 s by means of an automated pinch valves prefusion system and then rapidly washed out (green and red bar respectively). The experiment was performed at RT.– Habermacher et al 2016 (eLife, 5:e11050).
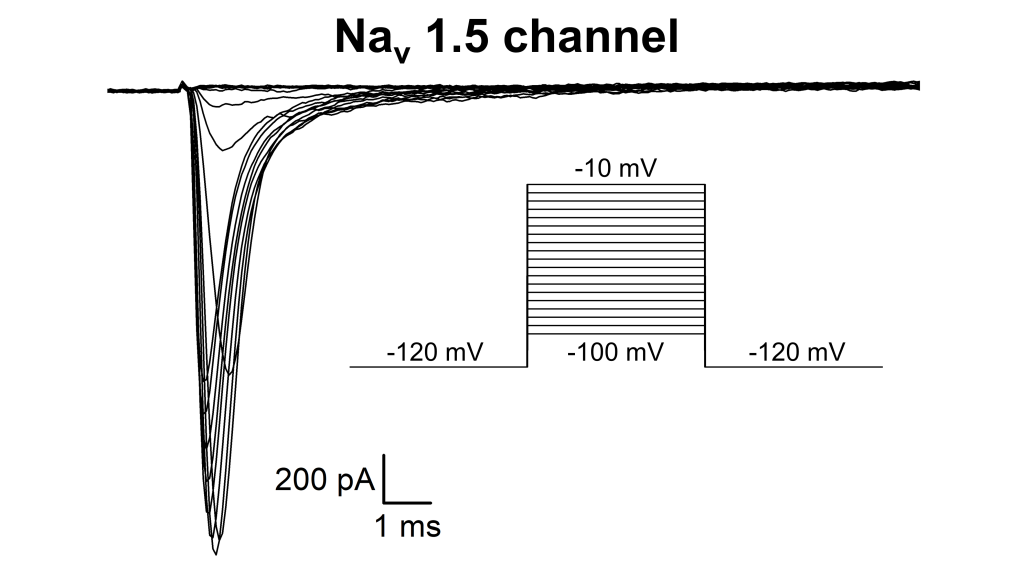 Voltage-dependent activation of Nav1.5 sodium channel expressed in HEK293T cells. Representative whole-cell current traces recordedfrom a HEK-Nav1.5 cell,using the indicated pulse protocol. Data were recorded at 10KHz bandwidth (Sampling Rate: 80 KHz).Both pipette and membrane capacitance were compensated. Voltage errors were minimized using 80% series resistance compensation. Pipette was filled with a solution containing (in mM): 135 NaCl, 4 KCl, 1 CaCl2,2 MgCl2, 10 HEPES, 20 Glucose, PH 7.4. Bath solution contained (in mM): 5 NaCl, 140CsCl, 4 Mg-ATP, 2 MgCl2, 5 EGTA, 10 HEPES, PH 7.4.
Voltage-dependent activation of Nav1.5 sodium channel expressed in HEK293T cells. Representative whole-cell current traces recordedfrom a HEK-Nav1.5 cell,using the indicated pulse protocol. Data were recorded at 10KHz bandwidth (Sampling Rate: 80 KHz).Both pipette and membrane capacitance were compensated. Voltage errors were minimized using 80% series resistance compensation. Pipette was filled with a solution containing (in mM): 135 NaCl, 4 KCl, 1 CaCl2,2 MgCl2, 10 HEPES, 20 Glucose, PH 7.4. Bath solution contained (in mM): 5 NaCl, 140CsCl, 4 Mg-ATP, 2 MgCl2, 5 EGTA, 10 HEPES, PH 7.4.
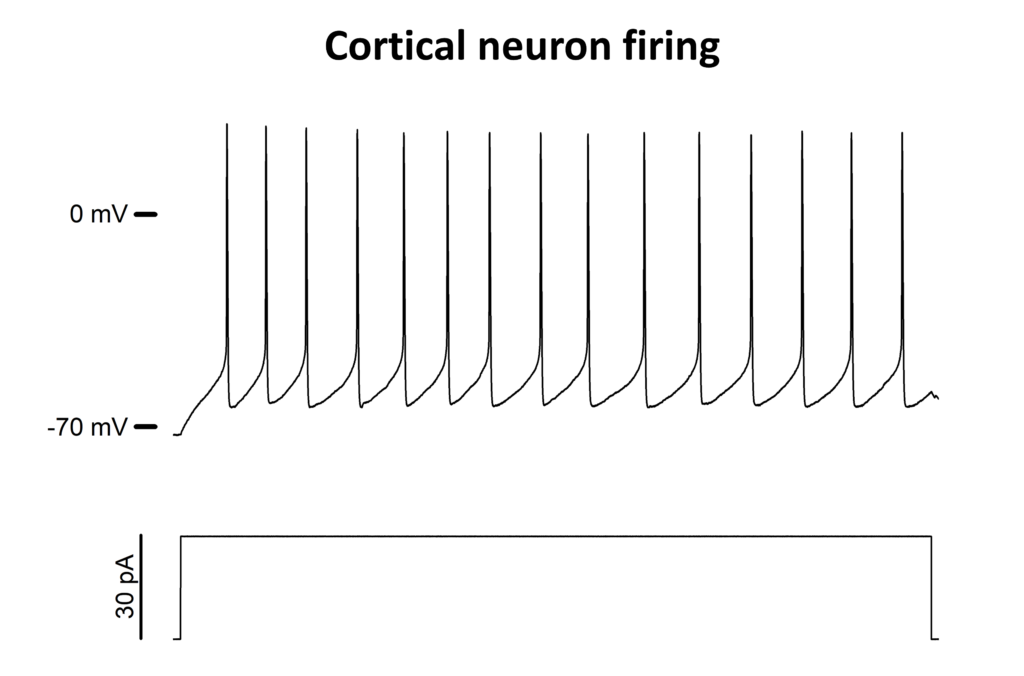
Whole-cell current-clamp recordings of a cortical neuron isolated from neonatal rat brains. After achieving the whole-cell configuration in VC mode, the amplifier was switched to the “true zero current mode” to measure the resting potential value. Afterwards, both the pipette neutralization and the bridge balance compensation were applied. The action potentials were finally recorded by injecting a series of current steps for 1 s (here is shown the most representative one) from the same holding potential of -70 mV. Data were acquired at 10 kHz SR and saved in .abf format.
– Data courtesy of Dr. A. Binda and Prof. I. Rivolta, School of Medicine and Surgery, University of Milano-Bicocca, Italy.
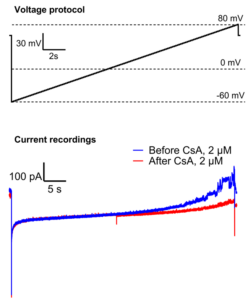 Mitochondrial permeability transition pore (mPTP) current recorded in excised patch clamp configuration. Ramp voltage protocol.
Mitochondrial permeability transition pore (mPTP) current recorded in excised patch clamp configuration. Ramp voltage protocol.
Mitochondria were isolated from neonatal mouse brain after ischemia reperfusion injury. Formation of mitoplast were induced by passive mitochondrial swelling without energy substrates. Recording was done in symmetric recording solution (150 mM KCl). Note the inhibition of mPTP channel activity after addition of Cyclosporin A (CsA).
– Data courtesy of Dr. Maria Neginskaya, Dept. of Medicine, Albert Einstein College of Medicine, NYC
Connecting your ePatch in a setup has never been so easy
Technical specifications Voltage Clamp
- Open input (RMS) noise: 300 fA rms @ 625 Hz – 1.5 pA rms @ 10 kHz – 12 pA rms @ 100 kHz
- Current ranges: ±200 pA (Gain 2.25 GΩ), ±2 nA (Gain 225 MΩ), ±20 nA (Gain 22.5 MΩ), ±200 nA (Gain 2.25 MΩ)
- Parametric voltage protocols for pulse generator in the range of ±500 mV
- C fast compensation range: 0-11 pF
- C slow comepensation range: C in 0-250 pF – Tau in 0-2500 μs
- R series compensation range: 0-25 MΩ
- Auto electrodes voltage offset fine compensation
- Zap pulse
Technical specifications Current Clamp
- Short circuit input (RMS) noise: 11 μV rms @ 625 Hz – 22 μV rms @ 10 kHz – 350 μV rms @ 100 kHz
- Parametric current protocols for pulse generator in the range of ±2.5 nA (res. 0.2 pA) and ±100 nA (res. 10 pA)
- Voltage range: ±700 mV
- Pipette neutralization range: 0-31 pF
- Bridge Balance compensation range: 0-40 MΩ
- True-zero current mode
Other technical specifications
- Available bandwidth between 625 Hz to 100 kHz
- Max sampling rate: 200 ksps
- Data output format .abf
- USB powered
- Dovetail or rod bar mounting
- Available 0-5V programmable digital output
- Size & Weight: 42 x 18 x 78 mm, 200 g
- Saponaro A, et al., “Structural determinants of ivabradine block of the open pore of HCN4”. PNAS, 2024
- Porro A, et al., “A high affinity switch for cAMP in the HCN pacemaker channels”. Nature Communications, 2024
- Saponaro A, et al., “Validation of the binding stoichiometry between HCN channels and their neuronal regulator TRIP8b by single molecule measurements”. Front Physiol, 2022 26;13:998176.
- Merseburg A, et al. “Seizures, behavioral deficits, and adverse drug responses in two new genetic mouse models of HCN1 epileptic encephalopathy”. Elife, 2022
- Mnatsakanyan, N et al., “Mitochondrial ATP synthase c-subunit leak channel triggers cell death upon loss of its F1 subcomplex”. Cell Death Differ, 2022
- Gutierrez BC et al., “Honeybee Brain Oscillations Are Generated by Microtubules. The Concept of a Brain Central Oscillator”. Front Mol Neurosci., 2021
- Porro A et al., “Do the functional properties of HCN1 mutants correlate with the clinical features in epileptic patients?” Prog Biophys Mol Biol., 2021
- Saponaro A et al., “Gating movements and ion permeation in HCN4 pacemaker channels”. Mol Cell., 2021
- Porro A et al., “Rational design of a mutation to investigate the role of the brain protein TRIP8b in limiting the cAMP response of HCN channels in neurons”. J Gen Physiol., 2020 7;152(9):e202012596.
- Porro A et al., “The HCN domain couples voltage gating and cAMP response in hyperpolarization-activated cyclic nucleotide-gated channels”. Elife., 2019
- Del Rocío Cantero M et al., “Actin filaments modulate electrical activity of brain microtubule protein two-dimensional sheets”. Cytoskeleton (Hoboken), 2020
- Porro et al., “Investigating the effects of lamotrigine on HCN channels: a test case for a new miniaturized patch-clamp amplifier”. Poster presentation at BPS meeting.
- Porro et al., “Helices D and E of HCN4 channel tune the cAMP responsiveness”. Poster presentation at BPS meeting.

Custom device development
With our custom ASIC design technology we can help you configure specific tools and solutions for your applications.
Let us know how we can help you design the tools and software you need.

